Answers can be found at www.pearsonhighered.com/floyd
Section 1–1
1. Every known element has
(a) the same type of atoms (b) the same number of atoms
(c) a unique type of atom (d) several different types of atoms
2. An atom consists of
(a) one nucleus and only one electron (b) one nucleus and one or more electrons
(c) protons, electrons, and neutrons (d) answers (b) and (c)
3. The nucleus of an atom is made up of
(a) protons and neutrons (b) electrons
(c) electrons and protons (d) electrons and neutrons
4. Valence electrons are
(a) in the closest orbit to the nucleus (b) in the most distant orbit from the nucleus
(c) in various orbits around the nucleus (d) not associated with a particular atom
5. A positive ion is formed when
(a) a valence electron breaks away from the atom
(b) there are more holes than electrons in the outer orbit
(c) two atoms bond together
(d) an atom gains an extra valence electron
Section 1–2
6. The most widely used semiconductive material in electronic devices is
(a) germanium (b) carbon (c) copper (d) silicon
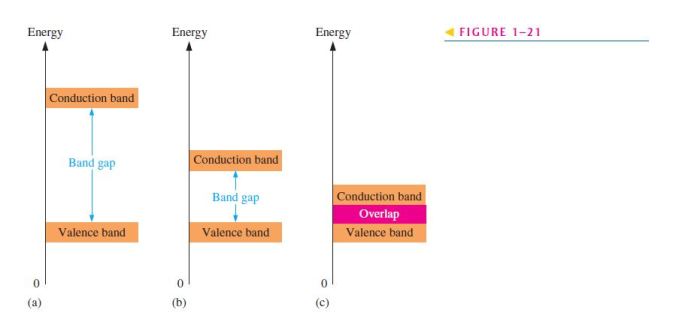
7. The difference between an insulator and a semiconductor is
(a) a wider energy gap between the valence band and the conduction band
(b) the number of free electrons
(c) the atomic structure
(d) answers (a), (b), and (c)
8. The energy band in which free electrons exist is the
(a) first band (b) second band (c) conduction band (d) valence band
9. In a semiconductor crystal, the atoms are held together by
(a) the interaction of valence electrons (b) forces of attraction
(c) covalent bonds (d) answers (a), (b), and (c)
10. The atomic number of silicon is
(a) 8 (b) 2 (c) 4 (d) 14
11. The atomic number of germanium is
(a) 8 (b) 2 (c) 4 (d) 32
12. The valence shell in a silicon atom has the number designation of
(a) 0 (b) 1 (c) 2 (d) 3
13. Each atom in a silicon crystal has
(a) four valence electrons
(b) four conduction electrons
(c) eight valence electrons, four of its own and four shared
(d) no valence electrons because all are shared with other atoms
Section 1–3
14. Electron-hole pairs are produced by
(a) recombination (b) thermal energy (c) ionization (d) doping
15. Recombination is when
(a) an electron falls into a hole
(b) a positive and a negative ion bond together
(c) a valence electron becomes a conduction electron
(d) a crystal is formed
16. The current in a semiconductor is produced by
(a) electrons only (b) holes only (c) negative ions (d) both electrons and holes
Section 1–4
17. In an intrinsic semiconductor,
(a) there are no free electrons
(b) the free electrons are thermally produced
(c) there are only holes
(d) there are as many electrons as there are holes
(e) answers (b) and (d)
18. The process of adding an impurity to an intrinsic semiconductor is called
(a) doping (b) recombination (c) atomic modification (d) ionization
19. A trivalent impurity is added to silicon to create
(a) germanium (b) a p-type semiconductor
(c) an n-type semiconductor (d) a depletion region
20. The purpose of a pentavalent impurity is to
(a) reduce the conductivity of silicon (b) increase the number of holes
(c) increase the number of free electrons (d) create minority carriers
21. The majority carriers in an n-type semiconductor are
(a) holes (b) valence electrons (c) conduction electrons (d) protons
22. Holes in an n-type semiconductor are
(a) minority carriers that are thermally produced
(b) minority carriers that are produced by doping
(c) majority carriers that are thermally produced
(d) majority carriers that are produced by doping
Section 1–5
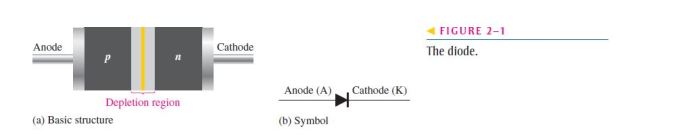
23. A pn junction is formed by
(a) the recombination of electrons and holes
(b) ionization
(c) the boundary of a p-type and an n-type material
(d) the collision of a proton and a neutron
24. The depletion region is created by
(a) ionization (b) diffusion (c) recombination (d) answers (a), (b), and (c)
25. The depletion region consists of
(a) nothing but minority carriers (b) positive and negative ions
(c) no majority carriers (d) answers (b) and (c)