- An atom is the smallest particle of an element that retains the characteristics of that element;
- Each of the known 118 elements has atoms that are different from the atoms of all other elements;
- This gives each element a unique atomic structure;
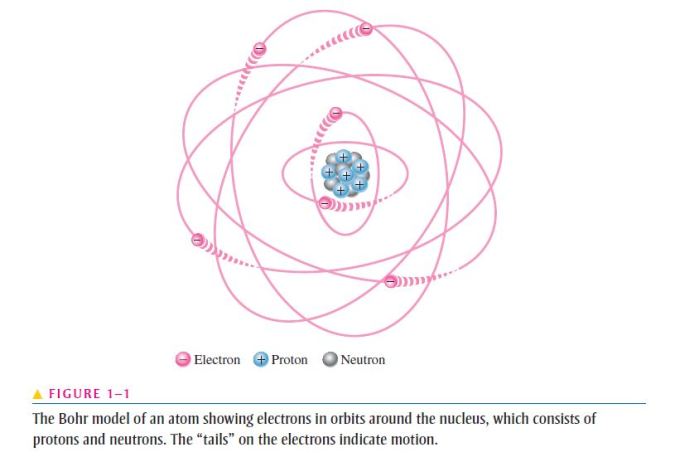
- According to the classical Bohr model, atoms have a planetary type of structure that consists of a central nucleus surrounded by orbiting electrons, as illustrated in Figure 1–1;
- The nucleus consists of positively charged particles called protons and uncharged particles called neutrons;
- The basic particles of negative charge are called electrons;
- Each type of atom has a certain number of electrons and protons that distinguish it from the atoms of all other elements;
- For example, the simplest atom is that of hydrogen,
which has one proton and one electron, as shown in Figure 1–2(a);
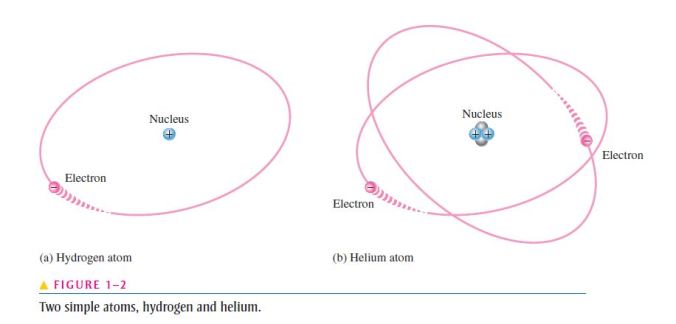
- As another example, the helium atom, shown in Figure 1–2(b), has two protons and two neutrons in the nucleus and two electrons orbiting the nucleus;
- All matter is composed of atoms;
- All atoms consist of electrons, protons, and neutrons except for normal hydrogen which does not have a neutron;
- Each element in the periodic table has a unique atomic structure;
- All atoms of a given element have the same number of protons;
- At first, the atom was thought to be a tiny indivisible sphere;
- Later it was shown that the atom was not a single particle but was made up of a small dense nucleus around which electrons orbit at great distances from the nucleus, similar to the way planets orbit the sun;