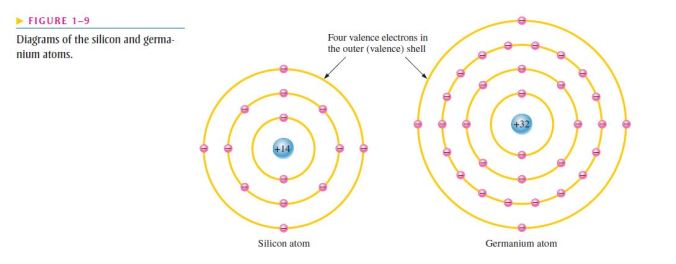
- The atomic structures of silicon and germanium are compared in Figure 1–9;
- Silicon is used in diodes, transistors, integrated circuits, and other semiconductor devices;
- Notice that both silicon and germanium have the characteristic four valence electrons;
- The valence electrons in germanium are in the fourth shell while those in silicon are in the third shell, closer to the nucleus;
- This means that the germanium valence electrons are at higher energy levels than those in silicon and, therefore, require a smaller amount of additional energy to escape from the atom;
- This property makes germanium more unstable at high temperatures and results in excessive reverse current;
- This is why silicon is a more widely used semiconductive material;
Covalent Bonds
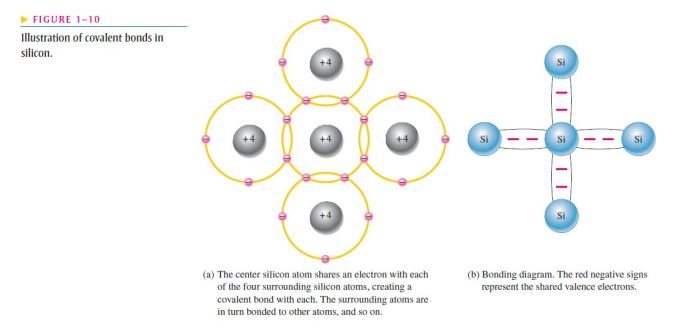
- Figure 1–10 shows how each silicon atom positions itself with four adjacent silicon atoms to form a silicon crystal;
- A silicon (Si) atom with its four valence electrons shares an electron with each of its four neighbors;
- This effectively creates eight shared valence electrons for each atom and produces a state of chemical stability;
- Also, this sharing of valence electrons produces the covalent bonds that hold the atoms together;
- Each valence electron is attracted equally by the two adjacent atoms which share it;
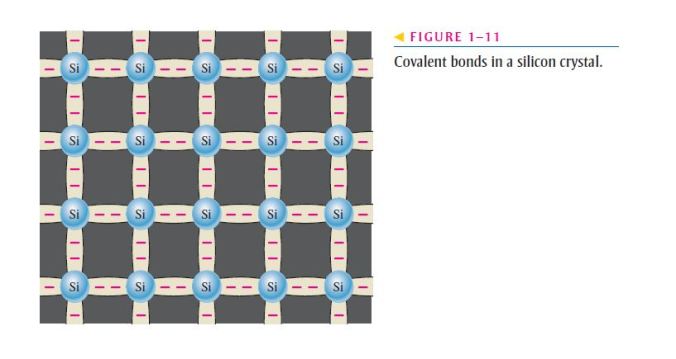
- Covalent bonding in an intrinsic silicon crystal is shown in Figure 1–11;
- An intrinsic crystal is one that has no impurities;
- Covalent bonding for germanium is similar because it also has four valence electrons;