Energy Levels
- Electrons orbit the nucleus of an atom at certain distances from the nucleus;
- Electrons near the nucleus have less energy than those in more distant orbits;
- Only discrete (separate and distinct) values of electron energies exist within atomic structures;
- Therefore, electrons must orbit only at discrete distances from the nucleus;
- Each discrete distance (orbit) from the nucleus corresponds to a certain energy level;
- In an atom, the orbits are grouped into energy levels known as shells;
- A given atom has a fixed number of shells;
- Each shell has a fixed maximum number of electrons;
- The shells (energy levels) are designated 1, 2, 3, and so on, with 1 being closest to the nucleus;
- The Bohr model of the silicon atom is shown in Figure 1–4;
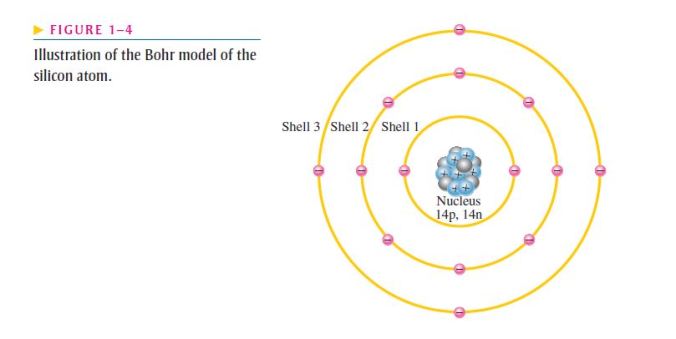
- Notice that there are 14 electrons and 14 each of protons and neutrons in the nucleus;
The Maximum Number of Electrons in Each Shell
- The maximum number of electrons (Ne) that can exist in each shell of an atom is a fact of nature and can be calculated by the formula:

- Where n is the number of the shell. The maximum number of electrons that can exist in the innermost shell (shell 1) is:
Ne = 2n2 = 2(1)2 = 2
- The maximum number of electrons that can exist in shell 2 is:
Ne = 2n2 = 2(2)2 = 2(4) = 8
- The maximum number of electrons that can exist in shell 3 is:
Ne = 2n2 = 2(3)2 = 2(9) = 18
- The maximum number of electrons that can exist in shell 4 is:
Ne = 2n2 = 2(4)2 = 2(16) = 32